Abstract
Introduction
Gene expression profiling (GEP) was the first assay to distinguish patients (pts) with DLBCL based on cell of origin (COO) into two subgroups, the germinal center B-cell like (GCB) with better outcome than the activated B-cell like DLBCL(ABC) (Alizadeh 2000). Due to disparate survival outcomes, there was enthusiasm to translate this into clinical practice with an immunohistochemistry-based method (Hans 2004). Despite widespread use, Hans classification has not led to significant changes in standard practice or development of novel therapies as it does not consider driver mutations and pathways which confer drug resistance (Roschweski 2014). Recently the LymphGen algorithm (Schmitz 2018; Runge 2021) and Dana Farber group classification (Chapuy 2018) have identified 7 and 5 subgroups of DLBCL, respectively, through the use of whole exome sequencing.
Albitar et al (2020, 2022) used machine learning to fit pts into four groups based on their overall survival (OS) and then selected biomarkers which can identify prognosis for those groups, developing DLBCL classification based on targeted RNA sequencing.
Pts in all mentioned analyses were treated with R-CHOP recognized as suboptimal in high risk DLBCL. We applied the Albitar et al classifier to a cohort of high risk DLBCL pts treated with dose-intense regimens (DIR).
Methods
Pts with DLBCL treated with DIR at John Theurer Cancer Center between 2010 and 2020 were identified. DIR included receipt of one of the following treatment regimens: R-HCVAD, R-CODOX-M/R-IVAC, or R-DA-EPOCH. Demographic, lymphoma and survivorship variables were collected. DIR was administered to patients if they had any of the following: high International Prognostic Index (IPI), double hit (DH), double expressor (DE), MYC translocation, or high Ki-67≥80%. Extracted RNA and DNA from fresh frozen paraffin extracted tissue from diagnostic samples were sequenced using targeted panels of 275 genes for DNA and 1408 genes for RNA. Cases were risk stratified using machine learning algorithm as previously reported into 4 groups: long survival in the long group (LL), short survival in the long group (LS), long survival in the short group (SL), and short survival in the short group (SS).
Results
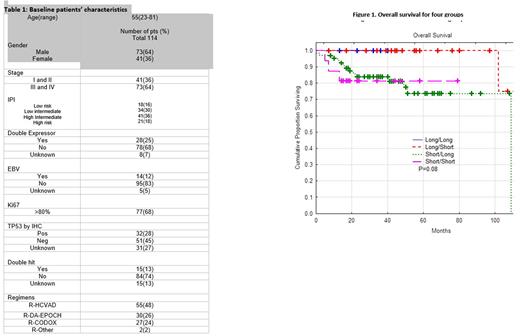
One-hundred fourteen pts were identified with characteristics that reflect high-risk status (Table 1). There were 31 pts in the long (L) groups (7 in LL, 24 in LS) and 83 pts in the short (S) groups (67 in SL, 16 in SS).
For the entire cohort, median(m)OS and mPFS were not reached. At 2 years, OS and PFS were 88% and 78%. An analysis based on DH, IPI, COO, and TP53 mutation status did not detect differences in PFS or OS. A trend towards worse 2-year OS of 50% was observed in DH-positive, SS pts (estimated(est.) p=0.29) After classifying pts into L and S groups, mOS and mPFS were not reached. The 2- year OS and PFS were 100% and 82% in L groups and 92% and 72% in S groups (est. p=0.01), which appears to be improved compared to a historical R-CHOP cohort. While the differences in OS and PFS between L and S groups is statistically significant, the separation between the curves has narrowed. Two-year OS and PFS by subgroups (LL, LS, SL, SS) were 100%, 100%, 82%, 80% (est. p=0.08) and 86%, 96%, 70%, 74% (est. p=0.08), respectively. When stratified by DIR, mOS and mPFS were not reached for all 4 groups. Two-year OS for the 4 groups (LL, LS, SL, SS) were: 100%, 100%, 96%, 96% (R-HCVAD); 100%,100%, 72%, 72% (R-CODOX-M/R-IVAC); and 100%, 100%, 74%, 68% (R-DA-EPOCH). Two-year OS was 100% for all regimens in the L groups compared to 98% (R-HCVAD), 74% (R-CODOX-M/R-IVAC), and 71% (R-DA-EPOCH) in the S groups. While there is a trend for worse OS and PFS in the S groups, specifically the SS subgroup, it is not statistically significant for all regimens.
Conclusion Our data shows that DIR improve the OS and PFS in high-risk DLBCL pts. mRNA sequencing algorithm applied to DIR-treated pts could not show a statistically significant difference among the molecular groups, although a trend towards worse outcomes was seen for the S groups. There was no difference in OS and PFS based on DH, IPI, COO, and TP53 mutation status. One subgroup which fared most poorly was the DH-positive, SS patients, indicating they require a completely different approach to frontline therapy. Future planned analyses will evaluate this subset of pts to identify potentially actionable biomarkers.
Disclosures
Feldman:Pharmacyclics LLC: Speakers Bureau; Celgene Corporation: Speakers Bureau; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Biotech, Inc.: Speakers Bureau; Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sakyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; SecuraBIO: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Speakers Bureau; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seagen: Speakers Bureau; Genomic Testing Cooperative: Other: Equity holder in privately-traded company. Albitar:Genomic Testing Cooperative: Current Employment, Current holder of stock options in a privately-held company. Ip:Pfizer: Honoraria; Seagen: Speakers Bureau; SecuraBio: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. Leslie:Abbvie: Consultancy; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Beigene: Consultancy, Honoraria, Speakers Bureau; Celgene: Speakers Bureau; Epizyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Karyopharm: Speakers Bureau; KITE: Consultancy, Speakers Bureau; Loxo/Eli Lily: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Consultancy; pharmacyclics: Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria. Pecora:Genomic Testing Cooperative: Other: Equity holder in privately held company; COTA: Other: Equity holder in privately held company; Celularity: Current equity holder in publicly-traded company. Goy:Seattle Genetics: Research Funding; Verastem: Research Funding; Karyopharm: Research Funding; lloplex: Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Clinical Advances in Hematology & Oncology: Consultancy, Honoraria; Rosewell Park: Consultancy, Honoraria; Infinity Pharmaceuticals: Research Funding; Hoffmann-La Roche: Research Funding; Genentech: Research Funding; Acerta: Research Funding; Physicians' Education Resource: Consultancy, Honoraria; Michael J. Hennessy Associates, Inc.: Consultancy, Honoraria; Medscape: Consultancy, Honoraria; AbbVie: Consultancy; Xcenda: Consultancy, Honoraria; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Steering committee, Research Funding; Vincerx: Honoraria, Other: Scientific Advisory Board; OncLive Peer Exchange: Consultancy, Honoraria; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MorphoSys: Honoraria, Other: Steering Committee, Research Funding; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee, Research Funding; Incyte: Honoraria, Other: Steering Committee, Research Funding; Elsevier Practice Update: Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Research Funding; Regional Cancer Care Associates: Current Employment; OMI: Current Employment; Cancer Outcome Tracking Analysis: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Genomic Testing Cooperative: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Resilience: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Other: Steering Committee, Research Funding; Bristol Meyers Squibb: Honoraria, Other: Scientific Advisory Board, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal